Researchers unveil the key regulator in protein production processes
MOLECULAR & COMPUTATIONAL BIOLOGY
9/2/2024
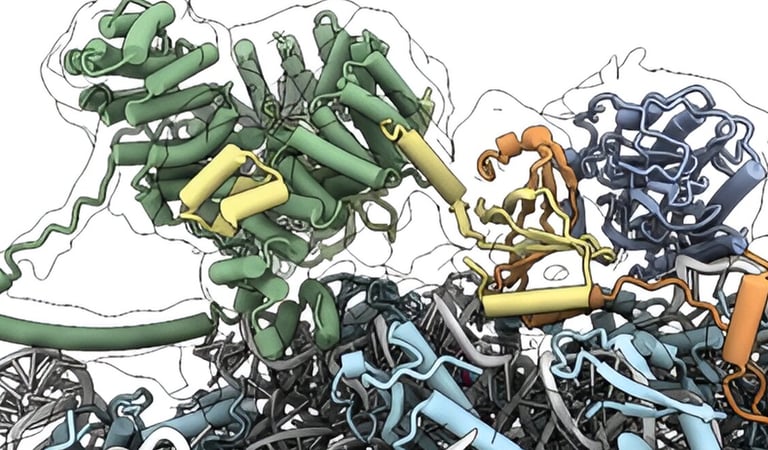
Approximately 40% of the mammalian proteome undergoes methionine cleavage and N-terminal acetylation, sequentially mediated by methionine aminopeptidase (MetAP) and N-acetyltransferase A (NatA). These two modifications occur concurrently with translation and are crucial for higher eukaryotic organisms. However, the interactions, activity, and regulation of these enzymes have not yet been fully understood. In a study published in Nature in August 2024, an international research team from Caltech, the University of Konstanz, and ETH Zurich revealed that the nascent polypeptide-associated complex (NAC) plays a key role in regulating the activity of MetAP and NatA. NAC forms a multi-enzyme complex with MetAP1 and NatA during the early stages of translation, precisely positioning the active sites of both enzymes to process newly synthesized proteins in the correct sequence and timing. The researchers also discovered that NAC can release the inhibitory interaction of the huntingtin yeast two-hybrid protein K (HYPK), a regulatory protein of NatA, thereby ensuring that N-terminal acetylation by NatA occurs during translation. In addition to providing a mechanistic model for protein processing in eukaryotic cells, these findings highlight how disruptions in NAC can impede protein processing by MetAP1 and NatA, potentially leading to serious diseases such as cancer and Parkinson's, and opening new opportunities for therapeutic development in the future.